
Because of the currency of some of the material presented below, students subject to rote testing based on the content of their textbooks are encouraged to review the Cautions Page before proceeding. It has been tailored separately for K-12, lower university and higher level students.
One of the few great shortcomings of biochemistry in the 20th Century was its failure to isolate and define the chromophores of biological vision. The treatise PROCESSES IN BIOLOGICAL VISION overcomes this difficulty. This page summarizes the situation developed in detail in Chapters 4, 5, 12 of that work. It shows that the historical concept of rhodopsin as a compound of retinol and opsin is archaic. A new concept is presented wherein rhodopsin is a chemical complex of Rhodonine and opsin. Even this complex by itself does not exhibit the properties of the visual chromophores. Only when complexed further with elements of the neural system does it exhibit the properties associated with the chromophores of vision.
This page will concentrate on the chemistry of the Rhodonines and provide links to separate pages defining the unique structural and spectral characteristics of these materials.
The four chromophores of biological vision are defined as the RHODONINES, a homologous chemical family based on a carboxyl-ion system and derivable from the simpler retinenes. They exhibit their unique spectral properties due to a quantum-mechanical resonance that is only apparent when the material is in the liquid crystalline state. Their performance is enhanced through their coating on a space-frame substrate. It is further enhanced by association with a de-excitation mechanism. A protein alone does not qualify as such a substrate nor does it provide a de-excitation mechanism!
The Rhodonines have avoided isolation and description for a very long time because of their:
This page will highlight each of the features of the Rhodonine family and point to more complete discussion elsewhere on this site. This is necessary because of the complexity of the molecules of this family and the interaction between its various unique components.
THE DESCRIPTION OF THE RHODONINESThe only rational way to describe the Rhodonines from a chemical perspective is using a large table. Such a table is provided in Section 5.2.5 of the treatise, PROCESSES IN BIOLOGICAL VISION. This table describes the Rhodonines as complex, conjugated organic molecules exhibiting triplet electronic states due to the incorporation of two oxygen atoms per molecule and quantum-mechanical resonance due to the conjugated condition existing between the two oxygen atoms. The resulting molecules each exhibit two distinct absorption spectra when in the liquid-crystalline state of matter, one of which is directly related to the level of the above conjugation. In the absence of any of these feature, the resulting material is not a Rhodonine and is not a chromophore of biological vision.
There are eight members of the family that constitute biological chromophores. All members are derivable biologically from the retinenes, four from the Vitamin A1 family and four from the Vitamin A1 family. Industrially, they are all derivable from the b-ionone family through the addition of a conjugated methine side chain. In both cases, oxygenation at specific locations is required.
The four members of the family derivable from Vitamin A1 are shared by all animals with a saline-based blood chemistry (virtually all marine and terrestrial chordates). The fresh-water based chordates (primarily fish) use the chromophores based on Vitamin A2. The euryhaline animals exchange their chromophore families during their life cycle.
The structure of the four saline based Rhodonines and the precursor, retinol1, are shown on a separate page. The fresh-water based set differ only in the bond arrangement within the b-ionone ring. This difference has negligible effect on the anisotropic absorption spectrum of the individual members of the family.
Casual review of the structure of the Rhodonines suggests they can be described as alcohols, aldehydes, ketones, retinenes and a variety of other names related to carbonyls and dicarbonyls. They can be described as retinoids. However, they are distinctly different from any other retinoids in the previously published literature. This is because of their distinct chemical resonance which contributes to their designation as a pH indicator. However, their key description is as a carboxylic-ion system with a variable degree of conjugation between the two oxygen atoms. Without recognizing the conjugated carboxylic-ion configuration, one cannot account for the chromophoric properties of the Rhodonines.
The fact that the Rhodonines will test positively for an alcohol, an aldehyde, a ketone and for a retinene (if the retinene test is not too specific) explains why there has been so much confusion in the vision literature. Most of the early so-called retinene tests actually assumed a retinene was present and tested for an alcohol or an aldehyde.
The fact that the Rhodonines are chemically resonant and act as pH indicators accounts for the confusion surrounding an additional set of tests reported in the literature that involved incubating the Rhodonines with some other material in an attempt to create rhodopsin.
The presence of oxygen in the carboxylic-ion system differentiates the Rhodonines from their close relatives, the cyanines and the merocyanines [5.5.5]. The presence of two oxygens in each molecule also differentiates them from the retinenes and most other retinoids.
Oxygen is a unique element that exhibits electron paramagnetic resonance. This property contributes to the unique presence of electrons in the triplet state in the Rhodonines. When excited, the ground state or n-electrons, which are already in the triplet electronic state, go directly into the triplet excited quantum-mechanical state associated with the p*-energy level. This is an unusual characteristic in quantum-chemistry. The fact the excited electrons exist in the triplet state accounts for the fact the Rhodonines exhibit very long excited lifetimes in the absence of an inter-system (between different chemical species) de-excitation mechanism[5.2.1]. They exhibit essentially no fluorescence and virtually no phosphorescence, particularly when in-vivo.
When in the liquid crystalline state, the Rhodonines exhibit a very large amount of energy level broadening due to the sharing of all of the triplet states within the crystal. This sharing must be accomplished within the constraints of the Pauli Exclusion Principle. The impact of this constraint will be discussed below.
The complex structure of the Rhodonines supports two separate and distinct absorption spectra associated with the visual spectrum. The first of these is described as a molecular resonance. In conjugated molecules, this resonance is frequently described as a dipole resonance to suggest that the molecule resonates as a rigid body. Such a resonance results in an isotropic absorption spectrum related to the physical length of the conjugated structure between the two massive terminal groups. This resonance is common to all Rhodonines. It exhibits a peak absorption between 495-502 nm depending on the environment of the molecule. It is this isotropic absorption spectrum that has historically been associated with rhodopsin (and both frequently and falsely with the scotopic luminosity function of the human eye). This absorption spectrum is not used in the functioning biological eye.
Lacking two heavy polar atoms, neither retinol, retinol in combination via a Schiff base, or any other retinene exhibits this absorption peak in the middle of the visual spectrum.
The second absorption spectrum related to the Rhodonines is anisotropic and exhibits a spectral peak unique to each member of the family. This absorption is related to a unique slow-wave structure associated with the triplet state electrons within each molecule. It will be described as an enhancement to the above molecular absorption. This enhanced absorption is due to an increased absorption cross section caused by the common resonant frequency between the triplet electrons within the molecule and the incident photons. As a result, this enhancement occurs at a frequency independent of that associated with the 495-502 nm peak described above. The enhanced peak is frequently more intense than the intrinsic dipole-molecular peak. [5.5.9] The wavelength of the enhanced absorption peak is a direct function of the level of conjugation between the two oxygen atoms of the Rhodonines. This mode of absorption is maximum when the incident photons are traveling parallel to the axis of the conjugate chain connecting the two oxygen atoms.
Both the dipole-molecular and the quantum-mechanical resonance modes of absorption are dependent on the physical length and rigidity of the molecular backbone of the molecule. In both cases, an all-trans- configuration is superior in terms of both the absorption cross section and peak wavelength compared to that of any cis- configuration.
Individual molecules of Rhodonine exhibit both the isotropic and the isotropic absorption spectra discussed above. However, the spectral bandwidth of the anisotropic spectrum is extremely narrow (similar to an absorption line in the spectrum of a pure molecular gas). This makes it difficult to detect and unsuitable for use in vision. However, if the molecules of a given Rhodonine are aggregated into a liquid crystalline structure, the rules of quantum mechanics require each ground state electron and each potential p*-level electron occupy separate energy levels. As a result, the aggregated mass exhibits a much broader absorption spectrum that is compatible with the requirements of vision. Assuming the liquid crystal is also planar in character, it will also exhibit a highly anisotropic absorption spectrum peaked in the direction of the conjugate axes of the molecules. These are the exact conditions observed in vision. Electron microscopy has demonstrated the molecules are aligned parallel to each other in such a liquid crystal coating on a suitable substrate and their axes are perpendicular to the surface of the substrate (in this case opsin) [4.3.2.4]. In the actual outer segment structure of the photoreceptor cells, this places the axes of maximum absorption parallel to the long axis of the outer segments and the direction of the incident radiation.
Because of the spectral broadening discussed above and the use of a large stack of chromophore coated disks in each outer segment, the absorption spectrum of each chromophore type is considerably broadened and the absorption cross section is considerably enlarged. These spectra are shown on the Rhodonine Spectra page.
When combined logarithmically by the circuitry of the visual system, these same spectra produce each of the psychophysical luminosity functions reported for human vision (when measured with a spectrometer with a spectral bandwidth of less than 10 nm).
Chemically, the Rhodonines are the chromophores of vision. However, their optimum performance is only achieved when they are complexed (not chemically combined) with other materials. Some of these materials are passive, like the protein opsin, while others are active living biological tissue, the dendrites of the neural system. This complexing is discussed in more detail on the rhodopsin page. It is discussed briefly below.
As developed in detail in Chapters 4 and 5 of PROCESSES IN BIOLOGICAL VISION, the concept of rhodopsin as the critical element of vision associated with the efficient sensing of photons in an efficient and timely manner requires the precise delineation of its characteristics and properties. These characteristics cannot be met by a simple chemical compound, partly because one of the elements of the complex is actually a physically separate living tissue. The rhodopsin complex consists of three uniquely distinct materials arranged in a unique physical arrangement:
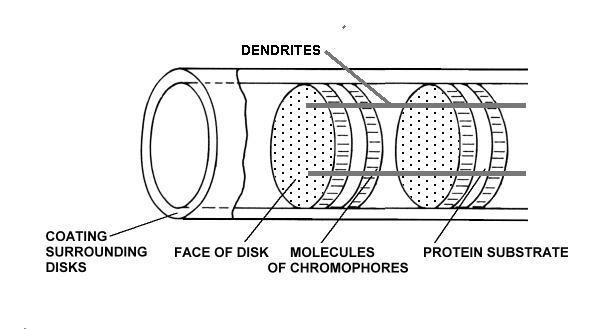
There are typically nine dendrites equally spaced around the outer segment and in quantum-mechanical contact with the chromophoric material. The complex is in close association with a fourth matrix, the IPM, that surrounds the individual disks of the outer segment.
The chromophoric material has been delivered to the disks of the outer segments of the photoreceptors via the IPM after being generated during physical transport from the liver and storage in the RPE. The space frame structure of the outer segments has been formed of opsin that has been secreted and then extruded into its final form by the glandular portion of the photoreceptor cell. The dendritic structure of the photoreceptor cell has been formed during genesis by the neural portion of the photoreceptor cell.
The protein material of each disk is completely passive and plays no role in the detection of light. The chromophoric material of each disk plays an active but conservative role in the detection of light. It accepts energy from the incident photons and transfers that energy to the dendrites of the neural system without any change in net energy to the chromophoric system. The dendrites of the neural system receive the quantum energy from the chromophores and translate it into free electrons within the electrical conduits of the neural system.
The only part of the rhodopsin complex that requires energy for its operation is the amplification function performed within the neural portion of the photoreceptor cells. This function is performed by an Activa located within the inner segment of the photoreceptor cell [Chap 12.5]. Since this function is physically located within the IPM, it is powered by an electrostenolytic mechanism provided by the IPM. Without electrical power from the IPM, the detection of photons by the photoreceptor cell is impossible. The materials associated with this electrostenolytic process are shown as the cylindrical coating surrounding the outer segment. This is the material that has frequently been mistaken for a membrane in the past. It is prominent in low resolution electron microscopy because of its electrical properties. However, under high resolution microscopy, it does note exhibit the distinct multiple layer structure of a membrane.
Rhodopsin as defined above is a chemical complex of Rhodonine and opsin that is non-functional in the absence of a de-excitation mechanism (the dendrites) and the associated electrical power source (the glutamate-based electrostenolytic mechanism).
The relatively crude chemical laboratory techniques used in the 1930-60's were not compatible with the study of the rhodopsin complex or the recovery of the chromophores of vision. Physical separation of the outer segments from the inner segments destroyed the de-excitation mechanism and insured the rapid bleaching of the remaining material. Application of various detergents destroyed the liquid crystalline state of the chromophores causing them to lose their anisotropic spectral characteristics associated with the individual short, medium and long wavelengths of vision. Finally, centrifugation destroyed the space frame structure required by the quantum-mechanics of the photon absorption process.
The above problems are easily overcome today through the use of an alternate but electrically active substrate on which the chromophores can be differentially re-crystallized within a oxidizer-free equivalent of the IPM environment.
Return to the website home page