
One of the few great shortcomings of biochemistry in the 20th Century was its failure to isolate and define the chromophores of biological vision. The treatise PROCESSES IN BIOLOGICAL VISION overcomes this difficulty. This page summarizes the situation. It shows that the historical concept of rhodopsin as a compound of retinol and opsin is archaic. A new concept wherein rhodopsin is a complex of Rhodonine and opsin is defined in detail.
Because of the revolutionary nature of some of the material presented below, students subject to rote testing are encouraged to review the Cautions Page before proceeding. It has been tailored separately for K-12, lower university and higher level students.
This page is divided into the following sections:
HISTORICAL CONCEPT OF RHODOPSINThe conceptual development of Rhodopsin has a long history. However, up to the present, this concept has remain tied to a conceptualization that was developed in the 1930-50's and never demonstrated to be correct in the laboratory. It was developed before the development of liquid crystal chemistry, organic semiconductor theory, much of quantum mechanics, modern dye chemistry, the electron microscope, and many other diagnostic techniques such as Electron Parametric Resonance.
This archaic concept was based on the concept of rhodopsin as a compound
In recent times, three paths of theoretical investigation have emerged based on the above putative rhodopsin. These must also be considered technically archaic.
None of the above fundamental assumptions concerning the molecular structure or physical arrangement of rhodopsin have been confirmed by subsequent laboratory investigations. It can be shown that none of them is representative of the true nature of the rhodopsin concept as a combination of a retinoid and a protein, opsin.
As will be shown below, there is substantial evidence that the outer segment is not an internal element of the photoreceptor cell at all and that it is not surrounded by any lemma.
The average lifetime of a human disk after the putative isometric change of at least one of its rhodopsin molecules is six weeks. Conversely, the number of photons arriving at a single photoreceptor disk is sufficient to excite all of the molecules of rhodopsin present within a matter of minutes or less. No plausible mechanism has been proposed as to how the excited molecules are transported to the RPE and back within such a short interval.
To appreciate the precise concept of rhodopsin as a complex based on the experiments provided in the literature, it is first necessary to review a number of simpler concepts and facts.
With the major advances made in chemistry during the last half century, it becomes important to carefully define certain conditions.
It is important to differentiate between a compound, a complex, a composition and a configuration based on their electronic relationship. It is no longer adequate to define a compound as a material made up of two or more elements, MEDLINE On-Line-Dictionaries. MEDLINE does not provide any definition for the words complex, composite or composition used as nouns.
As used here, a compound is a material made up of two or more elements held together by relatively strong electrostatic or Coulomb bonds, typically metallic (ionic) or non-metallic (covalent) bonds. Conversely, a complex consists of a group of materials or elements held together by weak electrostatic forces, typically hydrogen bonding. Within these definitions, the materials may exist in different electrical conformations known as configurations (stereoisomers). A composition, or conglomerate, is merely a mixture of materials and/or elements with negligible electronic interaction between them.
In the case of complexes involving unusual states of matter, it is not necessary for the weak electrostatic bonds to be uniformly distributed within the complex. Thus, it becomes impossible to write a precise chemical formula for these situations.
There is excellent electron microscopy available showing that the disk stacks that form the outer segments of photoreceptor cells are not surrounded by any kind of cell wall (lemma). Because of the limitations of the WEB, it is not feasible to reproduce this evidence here. It is available in Chapter 4 of PROCESSES IN BIOLOGICAL VISION. The individual Chapters are available for downloading in PDF format. More specific references to this material will be given by paragraph numbers in brackets [4.3]. The disk stacks are in fact extracellular.
It is now well established that the proteins forming the substrates of the disk stack are formed in the photoreceptor cells along with many other proteins and extruded by those cells into the Inter Photoreceptor Matrix (IPM)[4.3.2.2]. However, it is also well established that only minuscule amounts of retinol reach the photoreceptor cells. This retinol is used for normal cell growth and maintenance, not chromophore production.
There is now a vast and detailed literature describing how the putative retinoids of the chromophores of vision arrive at the disk stacks associated with, but external to, the photoreceptor cells via the RPE cells [7.1]. The so-called color granules within these RPE cells are the sites of storage of the individual chromophores.
There is compelling evidence that retinol is converted into a chromophore during its transport from the liver to the RPE cells of the retina [7.1.2.1].
There is now considerable experimental literature on the mechanisms by which the chromophores of vision are reclaimed in the phagocytosis of the disks of the outer segment [4.6.1 & 7.6] and redeployed through the IPM to activate new disks of the outer segments [7.1.2.3].
The rules of dye chemistry and the experience of the photographic industry in developing visual spectrum photo-sensitive materials suggests the actual structure of the chromophores of vision. By recognizing the unique properties of oxygen at the atomic and quantum-mechanical levels, additional proposals relative to the detailed structures of the chromophores can be made.
During the 1980's several sophisticated experiments were performed where nucleotides were injected into living animals and/or retinas. The movement of these nucleotides were recorded as a function of time. Both nucleotides formed from amino acids and nucleotides based on retinol were used. The results were spectacular. They showed that the amino acids formed the protein opsin within the inner segments of the photoreceptor cells. However, the labeled retinol never entered the photoreceptor cells. The retinoid was carried to the RPE cells via the blood stream and converted to the chromophores, Rhodonine. The Rhodonine was then transported to the disks by the binding protein, IRBP, via the interphotoreceptor matrix, IPM, without ever passing through the plasma membrane of the photoreceptor cells [4.6.4]. As a result, the chromophores of vision were deposited on the opsin substrates and thereby formed the complex conceptuallized as rhodopsin. There was no retinol present in the final complex. The opsin substrates were tracked until they were phagocytized by the RPE cells and the amino acids were returned to the blood stream associated with the choroid. The retinoids were not returned to the blood stream in significant amounts. After phagocytosis, they were stored in the RPE and reused in a continuous processing loop.
Based on these rules and guidelines, along with the above investigations, it is possible to define the complex known as rhodopsin in a much more detailed way than previously [4.5.1.4 & 4.5.3]. The most important findings relate to the quantum-mechanical structure of the chromophores of vision and their potential methods of de-excitation after excitation.
As developed in detail in Chapters 4 and 5 of PROCESSES IN BIOLOGICAL VISION, the concept of rhodopsin as the critical element of vision associated with the efficient sensing of photons in an efficient and timely manner requires the precise delineation of its characteristics and properties. These characteristics cannot be met by a simple chemical compound, partly because one of the elements of the complex is actually a physically separate living tissue. The rhodopsin complex consists of three uniquely distinct materials arranged in a unique physical arrangement:
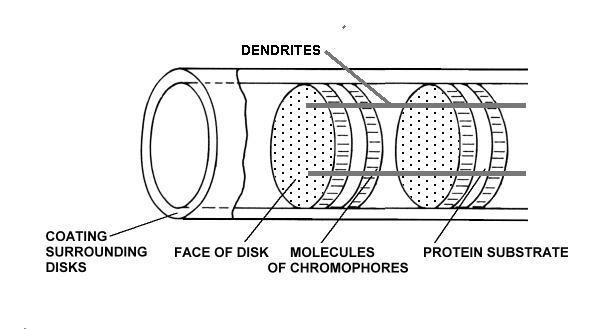
There are typically nine dendrites equally spaced around the outer segment and in quantum-mechanical contact with the chromophric material. The complex is in close association with a fourth matrix, the IPM, that surrounds the individual disks of the outer segment.
The chromophoric material has been delivered to the disks of the outer segments of the photoreceptors via the IPM after being generated during physical transport from the liver and storage in the RPE. The space frame structure of the outer segments has been formed of opsin that has been secreted and then extruded into its final form by the glandular portion of the photoreceptor cell. The dendritic structure of the photoreceptor cell has been formed during genesis by the neural portion of the photoreceptor cell.
The protein material of each disk is completely passive and plays no role in the detection of light. The chromophoric material of each disk plays an active but conservative role in the detection of light. It accepts energy from the incident photons and transfers that energy to the dendrites of the neural system without any change in net energy to the chromophoric system. The dendrites of the neural system receive the quantum energy from the chromophores and translate it into free electrons within the electrical conduits of the neural system.
The only part of the rhodopsin complex that requires energy for its operation is the amplification function performed within the neural portion of the photoreceptor cells. This function is performed by an Activa located within the inner segment of the photoreceptor cell [Chap 12.5]. Since this function is physically located within the IPM, it is powered by an electrostenolytic mechanism provided by the IPM. Without electrical power from the IPM, the detection of photons by the photoreceptor cell is impossible. The materials associated with this electrostenolytic process are shown as the cylindrical coating surrounding the outer segment. This is the material that has frequently been mistaken for a membrane in the past. It is prominent in low resolution electron microscopy because of its electrical properties. However, under high resolution microscopy, it does note exhibit the distinct multiple layer structure of a membrane.
Rhodopsin as defined above is a chemical complex of Rhodonine and opsin that is non-functional in the absence of a de-excitation mechanism (the dendrites) and the associated electrical power source (the glutamate-based electrostenolytic mechanism) Both the dendrites and the electrostenolytic mechanism depend on the electrical conductivity of the IPM. The two chemically distinct materials only share hydrogen bonds when the Rhodonine is coated onto the opsin. The Rhodonine is uniquely responsible for the absorption spectrum of the complex.
Based on the evidence discussed above, [4.6.4], the development and life cycle of rhodopsin as the active photosensitive element within the outer segments of vision can be described.The following figure illustrates this situation in detail but without a full discussion of those details. The details and citations are provided in Chapter 4 of PROCESSES IN BIOLOGICAL VISION which can be downloaded from this site.
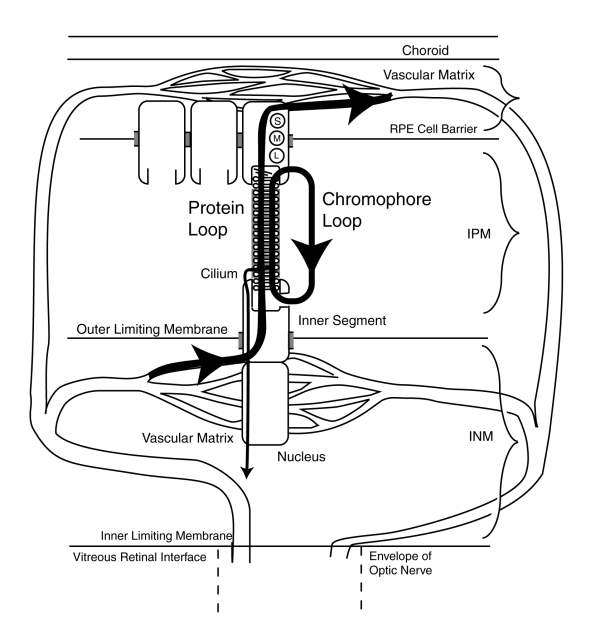
Note carefully that the electrical signal path through the cilium to the pedicle of the photoreceptor cell (shown by the vertical line with the small arrowhead) plays no role in the formation of either the opsin substrates or the chromophores deposited on the substrates. The amino based proteins are formed within the inner segment and secreted into the IPM filling the extrusion cup of the inner segment. IPM. There the protein, now opsin, is formed into disks that begin to travel toward the RPE. Upon reaching the RPE, this material is absorbed by the RPE cells and phagocytized, probably by hydrolysis, and returned to the bloodstream. Simultaneously, the chromophores have been formed within the RPE cells and are secreted into the IPM where they are transported by the binding protein known as IRBP to the extruding cup of the inner segment where they are deposited on the opsin substrates as a liquid crystalline coating. The material travels with the disks to the point of phagocytosis near the RPE cells where it is recovered and reused as shown. It is not released to the bloodstream unless it is no longer vital.
The relatively crude chemical laboratory techniques used in the 1930-60's were not compatible with the study of the rhodopsin complex or the recovery of the chromophores of vision. Physical separation of the outer segments from the inner segments destroyed the de-excitation mechanism and insured the rapid bleaching of the remaining material. Application of various detergents destroyed the liquid crystalline state of the chromophores causing them to lose their anisotropic spectral characteristics associated with the individual short, medium and long wavelengths of vision. Finally, centrifugation destroyed the space frame structure required by the quantum-mechanics of the photon absorption process.
The above problems are easily overcome today through the use of an alternate but electrically active substrate on which the chromophores can be differentially re-crystallized within a oxidizer-free equivalent of the IPM environment.
The chemical, structural and quantum mechanical details of the chromophores of vision, the Rhodonines, are presented on their own web page.
The four chromophores of biological vision are defined as the RHODONINES, a homologous chemical family based on a carboxyl-ion system and derivable from the simpler retinenes. [Chapter 5] They exhibit their unique spectral properties only in the liquid crystalline state. Their performance is enhanced through their coating on a spaceframe substrate. It is further enhanced by association with a de-excitation mechanism. A protein alone does not qualify as such a substrate nor does it provide a de-excitation mechanism!
Return to the website home page