Two distinct dynamic models are required to define the operation of the cochlea. The first describes the motions along the longitudinal length of the cochlea. The second, shown here, describes the propagation of the energy of a specific frequency across the gel surface of the tectorial membrane and its interception by the Outer Hair Cells.
The following animation incorporates the latest available information from the research community. This data clearly shows that the displacement pressure across the cochlear partition originates from within the cochlear partition and not from energy propagating within the perilymph1. The data also clearly establishes that the basilar membrane is not mechanically resonant along a lateral (or radial) dimension of the cochlear partition2.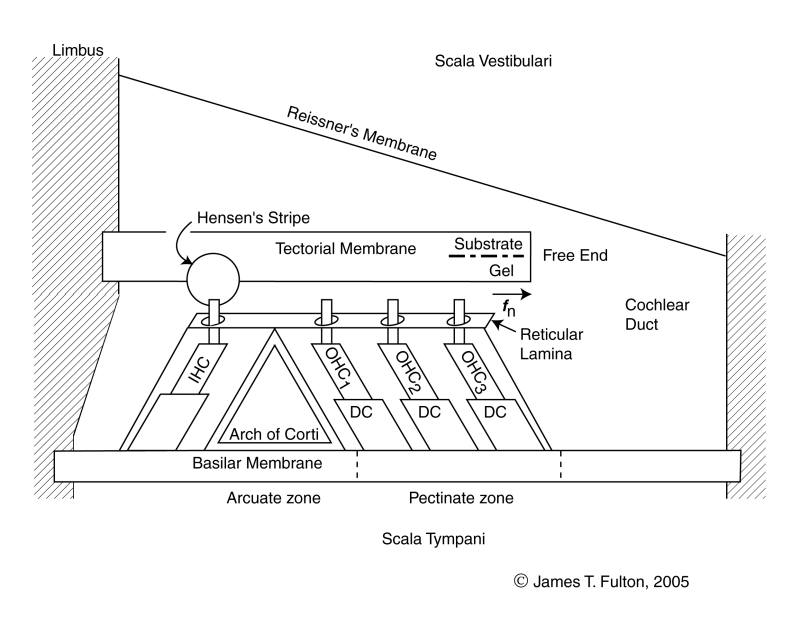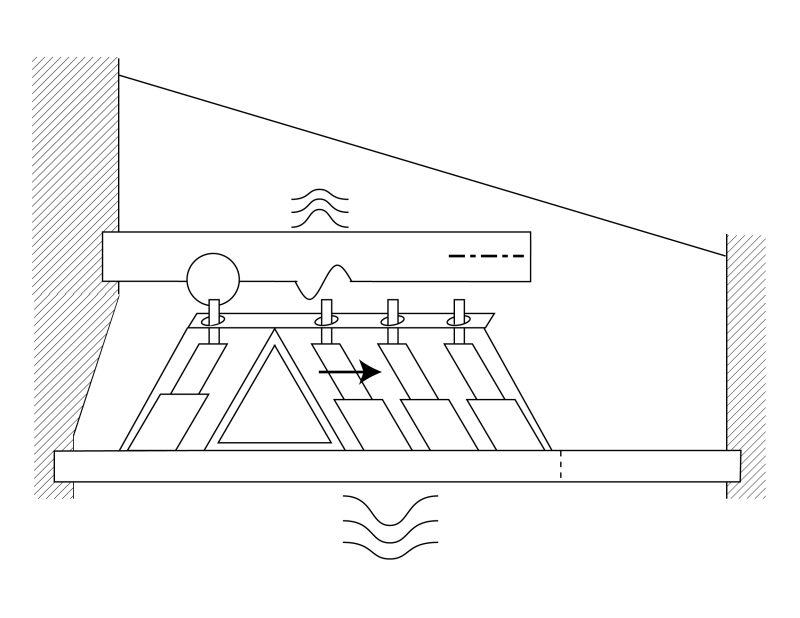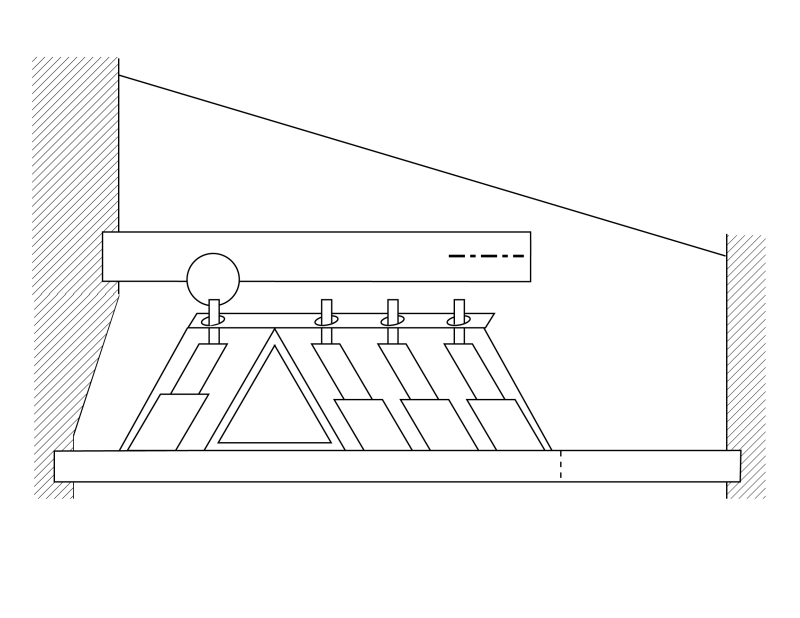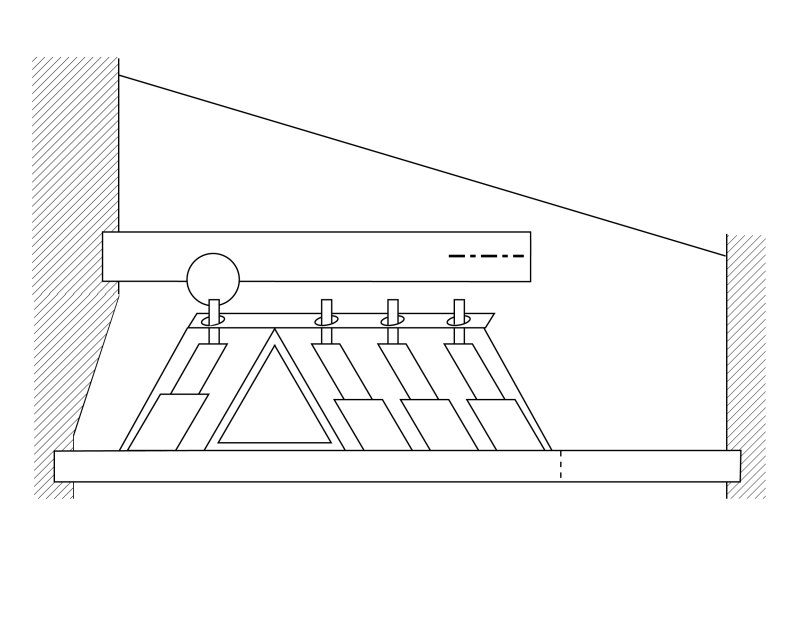
The energy to voltage converters in the above figure are not resonators. Frequency selectivity is by dispersion due to the Marcatili effect.
For the scope and top-level description of the SAW-based Electrolytic Theory of Hearing, see page 18 of Chapter 1 of the e-book "Processes of Biological Hearing"
The following material related to the longitudinal structural model of the cochlea is drawn from Section 4.3 of Chapter 4 of "Processes in Biological Hearing." A draft of this material can be accessed from the Home Page of this website.
The portion of the cochlear partition important in frequency selection is a lateral slice at an angle of 24 degrees to a radius. This is the angle at which the energy leaves Hensen's stripe. The width of the elementary slice is only 10 microns, the nominal width of the OHC that intercept the energy. This narrow slice, as a percentage of the length of the Organ of Corti (or more generally the cochlea) defines the ultimate frequency resolution of hearing. The location of the slice along the cochlea is a function of the frequency of interest.
As indicated in the longitudinal dynamic model, acoustic energy is introduced into the Organ of Corti as a surface acoustic wave traveling along one edge of the gelatinous surface of the tectorial membrane. The energy at a specific frequency is diverted and it crosses the gel surface of the membrane until it is intercepted by the Outer Hair Cells (OHC). The acoustic energy also travels across the gel surface as a surface acoustic wave with its amplitude transverse to the surface of the gel. The energy travels at a fixed velocity regardless of frequency.
The distance between the tectorial membrane and the basilar membrane is essentially fixed and their inertial mass resists any motion. Becauseof the ratio of the piezoelectric compliance of the OHC and the masses of the tectorial and basilar membranes, a majority of the energy is absorbed by the OHC. Only a residual amount is converted into motion of the two membranes.
The energy absorbed by the piezoelectric portion of the IHC is converted into an electrical voltage that is delivered to the neural system via the synapses of the IHC, a sensory neuron.
The recent laboratory data of Olson1 provides strong support for the following model. It has clearly established that the pressure gradient, near the basilar membrane during acoustic stimulation of the ear, is highest at the basilar membrane. The pressure in the perilymph decreases rapidly with distance from the basilar membrane.
The recent laboratory data of Nilsen & Russell2 provides strong support for the following model. They have clearly established that the basilar membrane is not a resonant structure in the area defined as the arcuate and pectinate zones. They showed the surface within these regions moved independently. They showed the overall waveforms generated following excitation of the structure were not sinusoidal. They also showed that the individual responses associated with each of these two area varied in phase with excitation frequency because the basilar membrane was not a purely resistive structure at that frequency.
The following animation is meant to describe how energy of a specific frequency is diverted from Hensen's stripe and redirected across the gel surface of the tectorial membrane to be intercepted by the Outer Hair Cells. The first frame of the animation is provided for orientation purposes. Callouts are provided to all of the relavent portions of the cochlear partition. It is best to show this animation in three separate subroutines.
The first subroutine will show a continuous traveling wave exciting the IHC and then being diverted across the gel surface in response to a continuous tone stimulation. Electrical signals (not shown) are generated at the IHC and each of the OHC.
The second subroutine is more detailed. It assumes stimulation by a short pulse at the critical frequency of this portion of the gel surface. Initially, the IHC is excited by the envelope of all of the energy in the applied stimulation remaining in Hensen's stripe at this longitudinal location. The energy at the critical frequency is then diverted across the gel surface. As in the longitudinal structural model, the gel surface is in contact with each of the cilia. The energy is shown exciting the cilia of the first, then the second and finally the third OHC. Because of the closeness of the OHC, these actions occur almost simultaneously (within a few microseconds). The tectorial membrane and the basilar membrane are acting as inertial masses and resist any vertical motion. As a result, most of the energy of the surface acoustic wave is converted into an electrical signal by the piezoelectric material in the IHC and OHC sensory neurons. The electrical output is shown by the horizontal arrows. A small amount of energy is transferred to the two membranes based on the ratio between the stiffness of the piezoelectric material and the dynamic properties of the two membranes. These small residual energies result in a small movement of the membranes and a corresponding pressure increase in the perilymph fluid next to each membrane. The motion of the masses may occur somewhat later than the time of OHC excitation.
The third subroutine is similar to the second. However, instead of focusing on the pressure buildup in the perilymph, it illustrates the physical movement of the basilar membrane in response to a pulse stimulus at the critical frequency of this part of the cochlear partition. Prior to any stimulation, the basilar membrane is nominally horizontal. As in the second routine, the pulse is first sensed by the IHC and results in a small residual vibration of the basilar membrane. Only the displacement of the membrane away from the gel surface is shown. The pulse then proceeds across the gel surface and stimulates the three OHC in rapid succession. As each OHC is stimulated, a small residual energy is transferred to the local area of the basilar membrane. This energy causes a vibration with a maximum displacement as shown. The overall effect is to cause the basilar membrane to teeter around the pivot point near the pyramid cell of the Arch of Corti nearest the first OHC.

The energy to voltage converters in the above figure are not resonators. Frequency selectivity is by dispersion due to the Marcatili effect.
Notice how the motion of the basilar membrane does not consist of sinusoidal components. The surface tilts relative to a pivot point much like a teeter-toter because of the stiffness of the membrane.Go to the first animation in the dynamic model chain.
Go to the roadmap of available animations of hearing.
Return to the hearing website home page.
1Olson, E. (1999) Direct measurement of intra-cochlear pressure waves Nature vol 402, pp 526-529 & citations
2Nilsen, K. & Russell, I. (1999) Timing of cochlear feedback: spatial and temporal representation of a tone across the basilar membrane Nature Neurosci vol. 2(7), pp 642-648