
|
CHEMORECEPTORS OF TASTE AND SMELL
|
Last update:
Activa™: See Citation Page
The biological organism employs a wide variety of chemoreceptors. This web page will only address the external chemoreceptors associated with the sensory modalities of gustation (taste) and olfaction (smell). These modalities employ very similar sensory strategies and circuits. The only significant differences between them are two. First, the precise chemical groups employed in the terminal structures (receptors) of their sensory receptor neurons. Second, the more extensive stage 2 signal processing performed in olfaction compared to gustation.
The Electrolytic Theory of the Neuron has formed a substantial foundation for the empirical work of Shallenberger and Acree on the "sweetness" of the sugars1. Their basic concept is illustrated below. It shows a gustatory receptor (a GR) within a taste bud coordinate bonding to a sweet tasting (typically sugar) molecule acting as a gustaphore (GU). They focused on the physical distance of 3.0 Angstrom between the Hydrogen (H) and the ligand at B. It is actually the distance between the two parallel hydrogen bonds (dotted lines) that is critical, in this case d = 2.6 Angstrom. This d-value will be employed extensively below.
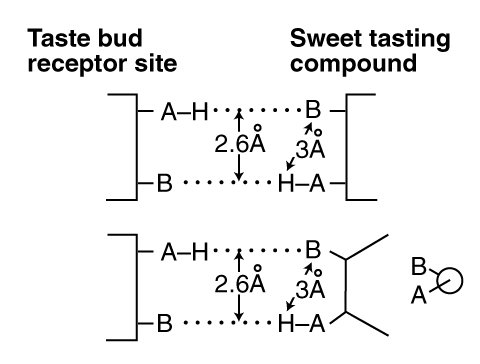
The bond pair shown is labeled "antiparallel" in modern chemistry to highlight the presence of the AH group at opposite ends of the two bonds.
The AH,B concept of coordinate chemical bond pairs is generally associated with Shallenberger. In the AH,B concept, two parameters are critically important. First, the distance between the AH (typically an hydroxyl ligand, OH) and the B (typically an oxygen ligand) elements of the stimulant. Second the magnitude of the dipole potential (related to the dipole moment but different) of the stimulant molecule.
The constituents of the AH,B relationship are not limited to hydrogen and oxygen. The A and B constituents can be any atom with shareable electron-pairs, such as oxygen, nitrogen, sulfur and potentially phosphorous. They can also be a p-bond of the benzene ring and under rare situations, an unsaturated carbon bond. The critical requirement is that these combined constituents satisfy the d-value required by the sensory receptor.
By expanding the framework, that Shallenberger and colleagues have already expanded to a degree2, it is possible to include both the gustatory and olfactory stimulants and receptors in the Theory.
Kier3 proposed adopting an additional critical dimension (X), the full concept being described as a three-point relationship between AH,B,X. This concept can account for the development of "super sweet" stimulants that generally do not contain any sugar ligand. They do however, generally contain a glucol group (1,2 cis-glycol). They frequently contain multiple gustaphore sites.
The super-sweeteners, which are generally man-made and only loosely related to the natural sugars, appear to involve this three-point interaction between the GU's and the GR's. This interaction involves a dual coordinate bond between a specific GR and the relevant GU's plus a modification of the dipole potential of the GR by the close proximity of an additional charge associated with the bulk of the individual GU.
The sensation space of the gustatory modality is considerably simpler than that of the olfactory modality, and gustation will be considered first. The reader may focus on the following sections as desired.
The Electrolytic Theory of the Neuron demonstrates there are only four distinct gustaphores. They are effective in the gustation modality because they exhibit two specific properties. They are selective for stimulants of a very specific stereochemistry, and they are able to measure the dipole potential of the stimulant (when captured stereochemically by the receptor). The dipole potential is a characteristic of asymmetrical organic compounds. The dipole potential is related to the dipole moment which is much more frequently measured in the laboratory. The dipole potential is easily measured with a Langmuir Apparatus, where the potential between the two sides of a monolayer film of the polar compound is present in the liquid crystalline state.
The gustaphores (stimulants) of taste exhibit specific stereochemical features as shown in the following figure.
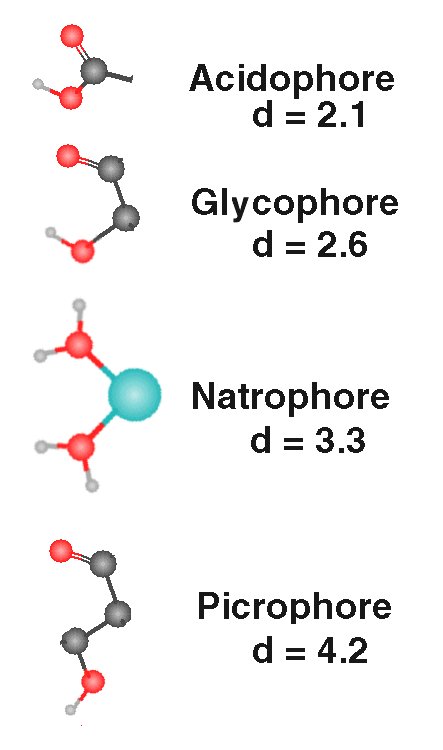
|
The "sour" acidophores are Lewis acids (organic) formed primarily by the carboxyl group. The diagonal distance between the two oxygen atoms is d = 1.4 Angrstom. A free hydrogen ion of a Bronsted (inorganic) acid is typically destructive of biological tissue. Hydrogen is not sensed by the gustatory system, but may be senses by nocioreceptors. |
| The "sweet" glucophores occur widely and most frequently consist of two oxygen atoms attached to two carbon atoms in the cis- configuration shown. The oxygen atoms are separated by a diagonal distance of d = 2.6 Angstrom. There is no requirement that a glycogen ring structure be present. | |
| The "salty" natrophores are formed by the complexing of the sodium ion with water. The natrophores consits of an oxygen from each of two adjacent oxygen atoms associated with two of the (typically six) waters of hydration complexed with a sodium atom. The diagonal distance between the two oxygen atoms is d = 3.3 Angstrom. | |
| The "bitter" picrophores are formed by two oxygen atoms separated by three carbon atoms in the configuraton shown. The diagonal distance between the two oxygen atoms is d = =4.2 Angstrom |
There have been many attempts to establish a fifth gustaphore, associated with the food additive monosodium glutamate and a number of similar substances. When dissolved in water, these highly soluble chemicals form a hydrated sodium ion (a natrophore) and the glutamate exhibits both a carboxyl group (an acidophore) and a glucol group (a glucophore). Thus, these materials stimulate at least two and frequently three of the analog gustaphore receptors.
Umami is distinctly not a separate and distinct sensation, or a separate and disticnt gustaphore.
The four sensory receptor channels of the gustatory modality (taste) report to the central nervous system (CNS) over separate and distinct neural channels (without significant stage 2 or stage 4 signal processing. Channels reporting independently are treated as if they were orthogonal mathematically. As a result, the gustatory sensation space can be described by the four corners of a three dimensional cube as shown below.
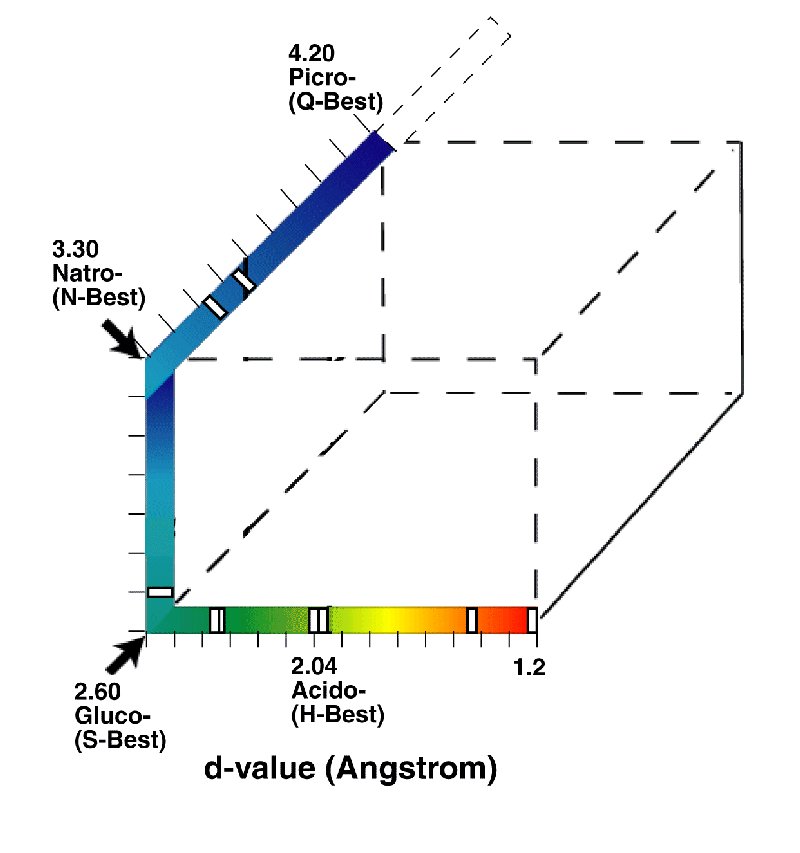
The space has been arranged based on the folding of a linear scale, the critical distance between the (typically oxygen) atoms of the gustaphores and corresponding molecular structure of the matching sensory receptors. The scale has been colored to show the similarity of the space to the similar space of the Chromaticity Diagram of Vision.
Well documented examples of some stimulants containing multiple gustaphores are shown as indices (white boxes) along the axes. These indices are suggestive of the relative dipole potentials of each gustaphore. Sensations of equal amplitude perceived by the CNS would be found at the midpoint of the distance between the two gustaphore locations.
While earlier investgators have shown this space as an equilateral tetrapod, this is less than accurate as the channels are independent and orthogonal. The angles between the primary gustaphores in sensation space are 90 degrees.
The Electrolytic Theory of the Neuron defines the gustaphores of the taste modality more clearly and precisely than historical practice. The following table describes these differences.
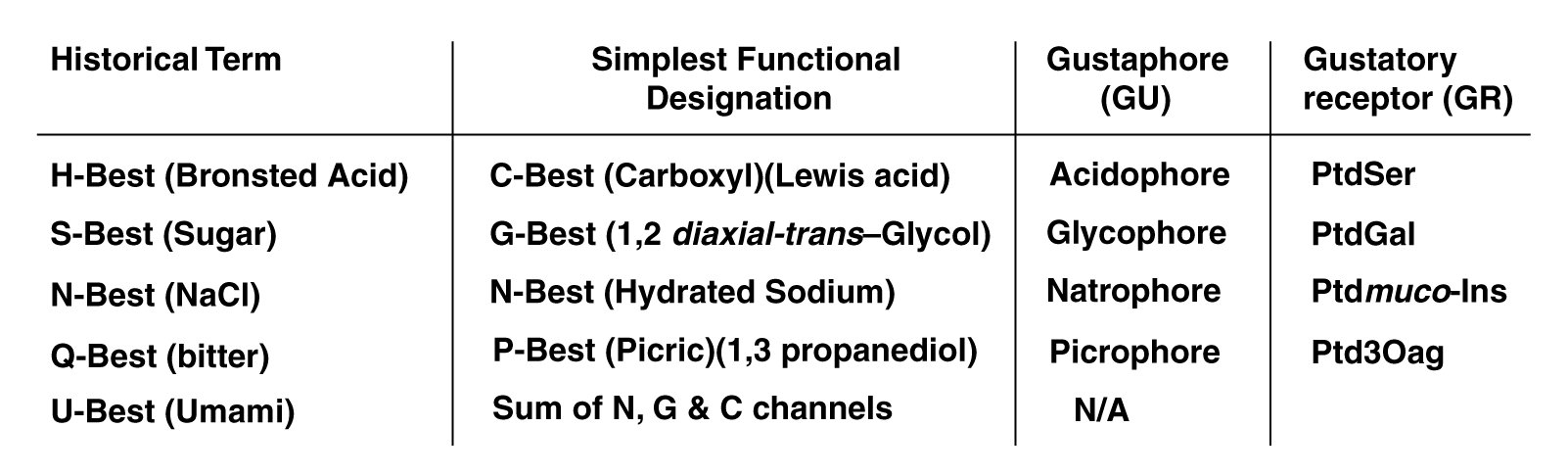
Historically, acidic has been used to describe a fundamental taste sensation without further definition. As noted, briefly above, most inorganic (Bronsted) acids are damaging to biological tissue. Only the Lewis acids of organic chemistry should be considered acidophores. The hydrogen ion alone is not capable of stereochemical bonding with the acidophore receptors. To be more precise, the term C-best can be used (signifying the carboxyl group) instead of the term, H-best.
The fact that many chemicals that are not technically sugars taste sweet, suggests the term G-best is more appropriate when speaking in a technical context. All chemicals containing the glucol group are glucophores. A glucol group is defined more stereo-specifically than the similar glycol group. Glucol is a 1,2 cis-glycol or a partially dehydrogenated derivative. The glucol group must be exposed and be capable of stereo-chemical complexing with the glucophore receptor. Some sugars do not satisfy this criteria and exhibit little or no "sweetness".
"Saltiness" is an ancient descriptor. However, the Electrolytic Theory of the Neuron clearly demonstrates a material need not be a salt in the vernacular. It need only provide a sodium ion when dissolved in water. It is the sodium ion when complexed with water that is sensed by the natrophore receptor. N-best remains the technical label best suited to this class of gustaphores.
The "bitter" taste sensation has always been difficult to describe with precision. Quinine in solution has always been used as the classic example of bitterness. However, many other compounds taste bitter. These compounds all contain the picrophore described above. The more general P-best provides a better lable for this class of compounds than does Q-best.
Throughout the 20th Century, various Japanese investigators have attempted to establish a fifth gustaphore of the taste modality, centered on the role of monosodium glutamate. Yamaguchi has been the leading investigator of umami. He has prepared the most extensive study of this putative sensation, including extensive lists of chemicals believed to involve the umami sensation4,5. This work clearly establishes that umami is not an independent class of gustaphores, nor is it a primary neural sensation. The perceived sensation is a mixture of the three primary sensations, C-best (acidiphore), G-best (a sweet glucophore) and N-best (a hydrated sodium natrophore).
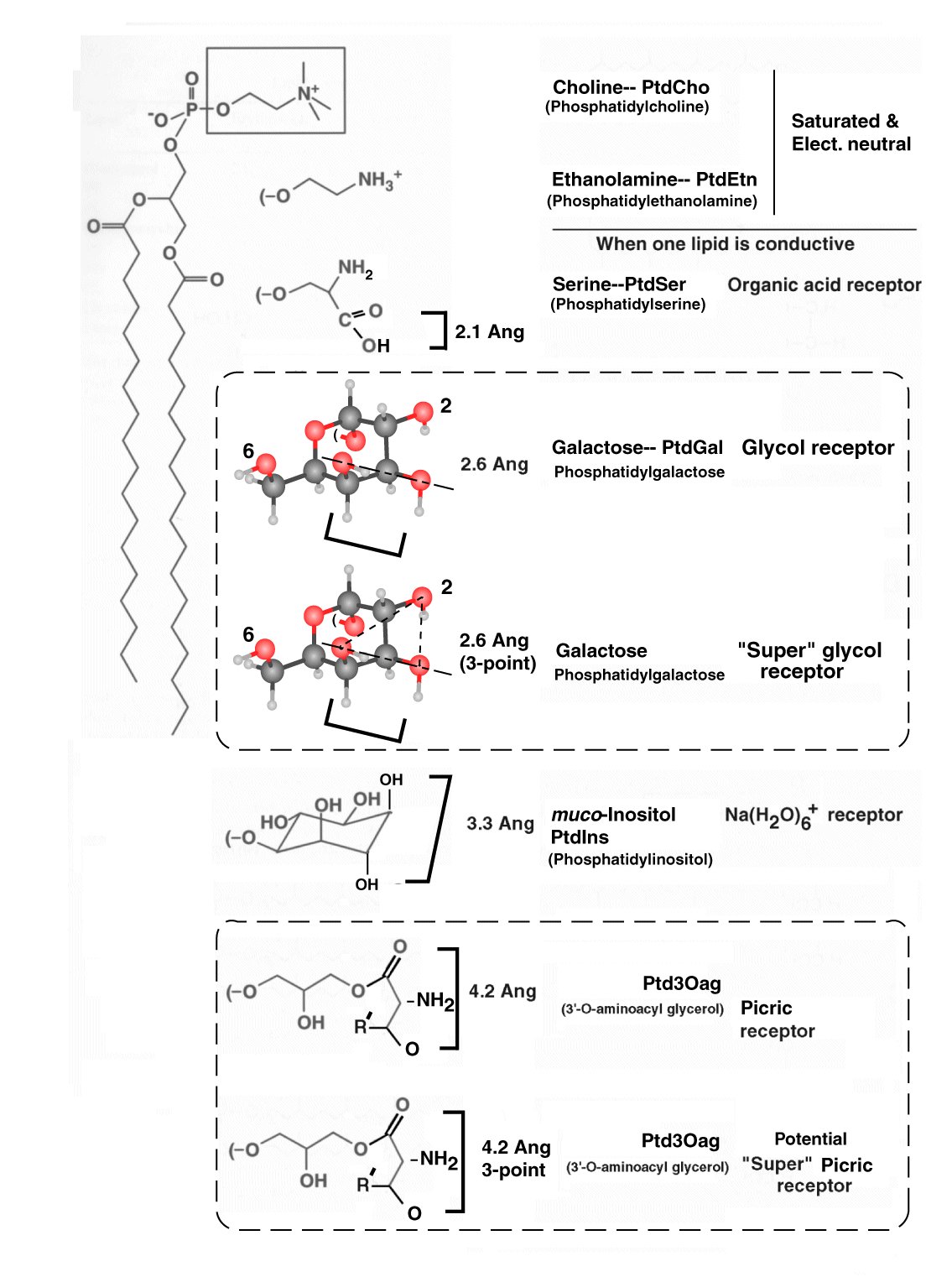
The chemical receptors (GR's) of the gustatory sensory neurons are complex phospholipids. Most of them are well represented in textbooks on Biochemistry. They are typically present in an identifiable and specialized region of the outer bilayer of type 2 lemma. The receptor molecules are present in a liquid crystalline structure of highly specific stereochemistry.
The four proposed receptors of gustation are phospholipids:
The following figure, taken from the text, is too complicated for the immediate purpose but it suggests how the chemical receptors arose and how they pair with their gustaphores. Phosphatidylcholine (PtdCho) and phosphatidylethanolamine (PtdEtn) are the phospholipids found in the majority of type 1 lemma. They must be modified to provide the necessary stereochemical and dipole potential measuring capabilities required. The square brackets indicate the orientation of the gustaphore required in order to stereochemically coordinate with the receptor. Section 8.5.1 of Chapter 8 provides the complete explanation of this figure.
The number of sensory receptors of smell appears to be open ended and to vary significantly among species, and even individual within a species. Individuals suffering total anosmia (the inability to perceive odors) are rare. However, those suffering specific anosmias (the inability to sense a specific odor generally sensed by other subjects) are common, especially when compared with some with the best olfactory capabilities. The number of documentable olfactory receptors appears to be between 15 and 25.
Estimates of hundreds to thousands of receptors in the recent literature based on very early genetic studies are not based on an understanding of the sensory modality. While there may be thousands of genes related to the olfactory system (many associated with the structural features of the Olfactory elements of the modality), and thousands of olfactory stimulants, these are all associated with a much smaller set of individual sensory receptors and a very efficient matrixing function located within the glomeruli of the olfactory bulb.
The sensing function of the olfactory modality employs the identical techniques and sensory neural circuitry as that of gustation. The difference is in the specific chemical groups attached to the terminal elements of the sensory neurons as the sensory receptors.
Evolution and the concept of ecological niches appears to play a significant role in understanding the olfactory sensory modality. While the utility of a natrophore (sodium sensory) receptor among the fish appears limited, except possibly for the catadromous fish like salmon during parts of their life, since they live in a medium of high sodium ion concentration. The availability of an acidophore (available among the marine animals) would clearly be of utility to early terrestrial animals in searching for food over larger radii. As the terrestrial animals began to evolve, additional sensory receptors associated with food acquisition appears highly useful. More recently, the evolution of the flowering plants suggests further evolution of sensory receptors of the more complex organics the flowers release.
The olfactophores of the olfactory sensory modality can be organized and understood using the framework developed by Shallenberger and Acree beginning in the 1960's. The following table is a draft being developed for the formal text and is subject to change and additional analysis leading to better d-values. However, the chemistry of the olfactophores is clear. They follow the same rules as initially described by Shallenberger and Acree. The family is much larger because of the number of chemical structures exhibiting sufficiently low vapor pressure to have significnat presence in the ground level terrestrial atmosphere.
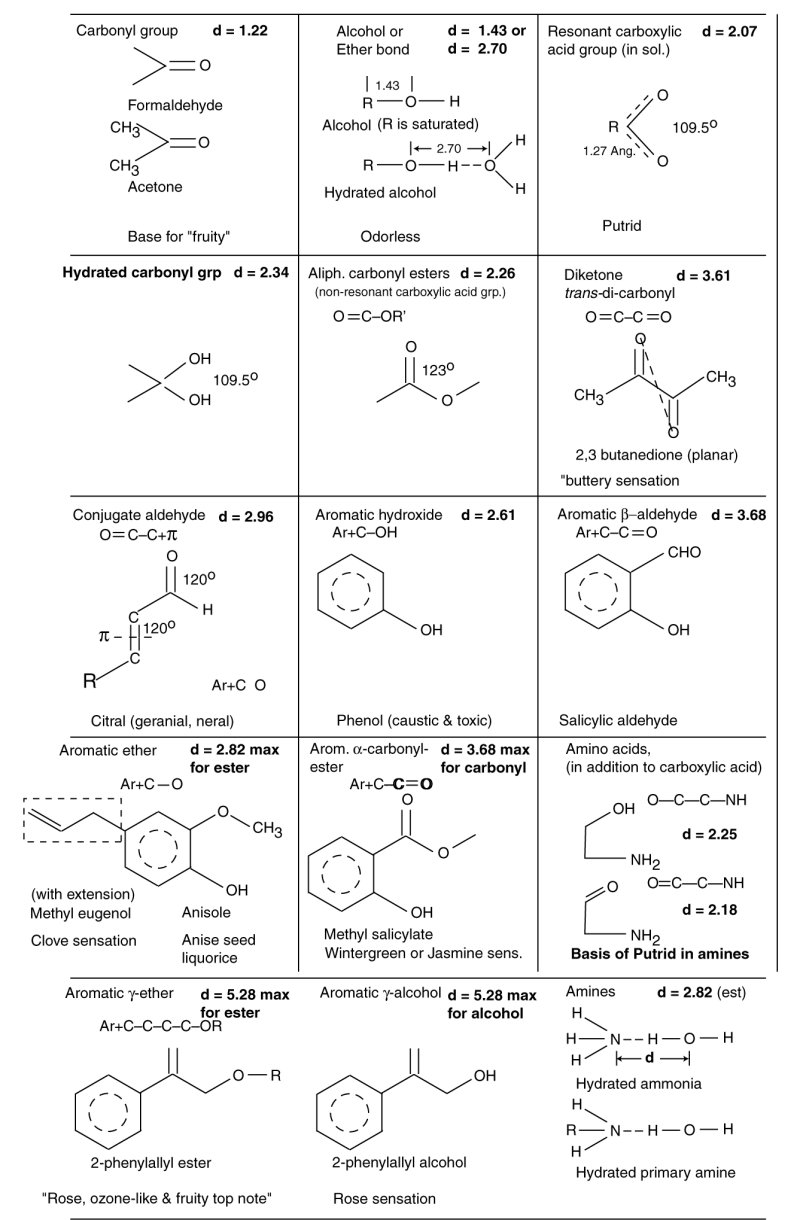
The family of olfactophores is expanded considerably by the introduction of aromatic rings into the previously simple aliphatic structures. The ring can act as one of the charge centers of Shallenberger and Acree. The charge is not typically located at the center of the ring. Therefore, determining the precise d-value of an aromatic-aliphatic olfactophore is difficult. In addition, the total number of olfactory stimulants includes many exhibiting multiple individual olfactophores. The potential number of olfactophore combinations appears limitless.
The sensory space of olfaction appears to vary significantly among mammals based on their ecological niche. In humans, there are 23 identified sensory receptor channels entering the glomeruli of the olfactory bulb from the olfactory epithelium. There may also be a few more channels associated with the vomeronasal channels (pheromone detection related to sexual function). There may be a few channels yet to be identified. The glomeruli is the site of significant stage 2 signal processing. The number of distinct channels projecting from the glomeruli to the Central Nervous System (CNS) remains to be determined at present. However, it is obvious that the relevant distance in d-value space exceeds that from d = 1.4 Angstrom to at least d = 5.28 Angstrom.
The proposal that there are thousands of individual olfactory sensory receptors (OR's), based on a cursory reading of less than 5% of the genetic code associated with protein synthesis, is not supported here. Thousands of individual olfactophores are easily sensed using less than three dozen OR's, matched to the chemical families identified above.
The best estimates of the odor receptors and their performance parameters are shown in the following figure. As in the earlier figure for the gustatory receptors, the d-values are based on the Jmol files of the Royal Society of Chemistry (RSC). These files have recently been abandoned by the RSC due to their lack of curation. However, they are the best data available at the current time
Also shown in this figure are the first two sensory bands associated with the vodorophores of Oskonation, the sensing of species and gender among the animals. Oskonation, as a subject, will be added to this web page in the near future.
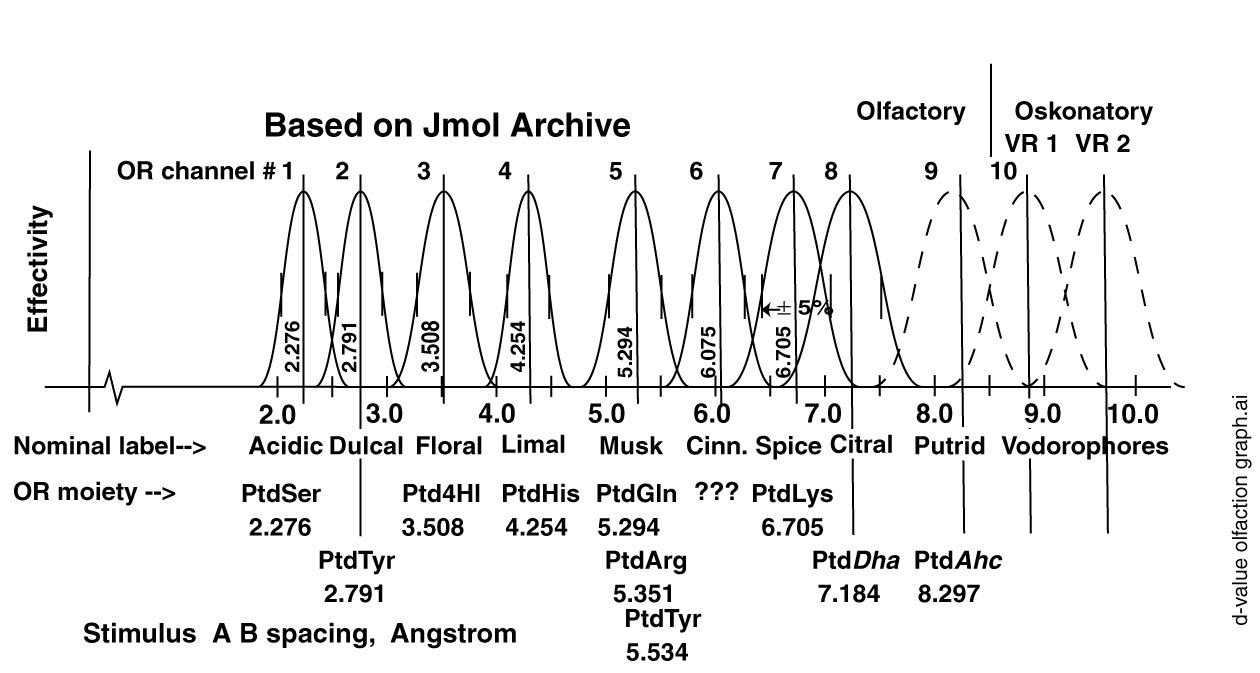
Like the super-sweeteners, the super-bitters appear to involve a three-point interaction between the OL's and the OR's. This interaction involves a dual coordinate bond between a specific OR and the relevant OL's plus a modification of the dipole potential of the OR by the close proximity of an additional charge associated with the bulk of the individual OL.
Return to Chemoreceptor main page
1. Shallenberger, R. & Acree, T. (1967) Molecular theory of sweet taste Nature vol 216, pp 480-482
2. Shallenberger, R. (1996) The AH,B glycophore and general taste chemistry Food Chem
vol 56(3), pp 209-214
3. Kier, L. (1972) A molecular theory of sweet taste J Pharm Sci vol 61(9), pp 1394-1397
4. Yamaguchi, S. (1979) The umami taste In Boudreau, J. ed Food Taste Chemistry. Washington, DC: American Chemical Soc Chapter 2
5. Yamaguchi, S. & Komata, Y. (1987) Independence and primacy of umami as compared with the four basic tastes Annals NY Acad Sci vol 510, pp 725-726