
 | ... | FALSIFICATION of the |
... |
The Chemical Theory of the Neuron is unsustainable in the light of modern technology. While the majority of electrophysiologists now recognize its inapplicability and have introduced "pores" and "gates" to avoid the porosity problem intrinsic to the Theory, it is still taught widely at the University Level and permeates academic papers at the conceptual level. The INTERNET has further complicated this problem by supporting the endless proliferation of web pages manipulating the diffusion equations of Nernst, Donnan, and Goldman purporting to apply to the fundamentally impermeable plasma membranes of all neurons.
The Theory emerged prior to the start of the 20th Century during a period of;
As a result, the Chemical Theory of the Neuron grew with the empirical data base within the histological and physiological laboratories of the early 20th Century. A majority of the expansion of the Theory occurred in the first half of the 20th Century;
This page will summarize the problems with the Chemical Theory of the Neuron and the reasons it is falsified by the greater knowledge base at the start of the 21st Century and the laboratory investigations of the last half of the 20th Century. Support for this page will be found in Chapter 1 of the text, "The Neuron and Neural Systems; A 21st Century Paradigm" available on this website.
The basic premise of the Chemical Theory of the Neuron is that the lemma of a cell is a semipermeable membrane to a variety of simple molecules and ions, both ionic and non-ionic in character, when in solution. Following the very early work of Nernst (1890's), efforts were made to describe the static internal potential of the neuron relative to its external environment based on the Nernst Equations. The early results were very limited and achieved primarily by assuming arbitrary concentrations of selected chemical species on each side of the lemma (and ignoring the presence of all other species).
To aleviate problems with application of the the Nernst Equation, Donner addressed the static situation again and implemented a modified equation in the (xxx)'s. He was able to rationalize the electrical potential within the axolemma of his selected neuron and the concentrations within and outside of the cell, but was unable to generalize the situation.
Goldman attempted to modify the Donner equation further in order to rationalize the static internal potential of additional neurons measured in the laboratory.
In the 1940's, Hodgkin, then Hodgkin and Huxley, and then Hodgkin, Huxley and Katz addressed the dynamics of the neuron as they understood it. Their generic neuron consisted of a single active chamber surrounded by an axolemma. The assumption was that the axolemma itself must be the active element. Hodgkin attempted to develop a cursory dynamic theory in 1949 based on his work with crustaceans. Hodgkin and Huxley reported on extensive laboratory investigations on the "giant axon" of the squid, Loligo in the 1950's. In hindsight, their work suffered from a variety of shortcomings that still plague the community today. The chemical aspects of these problems are addressed below. The problems associated with computational modeling of their framework are addressed in a separate document, the Falsification of the computational models of the Hodgkin and Huxley neuron.
Throughout the first half of the 20th Century, efforts were made to identify the dominant chemical species associated with the polarization of the neuron in the in-vivo environment. During this period, the size of the dendrites and the unknown podite structures made it very difficult if not impossible to evaluate the potentials of the dendroplasm and podaplasm. The prevalent concept was that the higher concentration of sodium ions outside of the neuron and the higher concentration of potassium ions within the axoplasm were probably the dominant species controlling the quiescent potential between the extra-neural matrix and the axoplasm.
Initially, Hodgkin and Huxley only discussed inward currents and outward currents in their attempts to describe the operation of the neuron. They later used the euphemisms, inward sodium current and outward potassium current in accordance with the prevalent beliefs. With their widely heralded work, these labels were shortened to the sodium current and pottasium currents (and others) without any consideration of the role of electrons in electro-chemistry (and without recognition of Benjamin Franklin's error in assuming positive charges flowed from the positive terminal of a battery through an external circuit to the negative terminal. The fact is the only electrical current flowing within an electro-chemical environment is the electron charge flowing from the negative terminal to the positive terminal of a battery. It is this charge flow that is measured in all laboratory environment not involving quantum-mechanical semiconductors. A pseudo-positive charge flow (hole current flow) does exist in quantum-mechanical semiconductors but this was unknown until the 1950's. Quantum-mechanical semiconductors do include (including many molecules and specifically liquid crystalline molecular species.
A major problem with the Chemical Theory of the Neuron has been the remarkably small amounts of electrical charge involved in neural operations. For millivolt changes in the proposed axoplasm potential and pico-farad capacitances associated with the axolemma of realistic size neurons, the movement of only micro-micro-micro-moles of ions or electron charges through the axolemma are required. Such small amounts remain unmeasurable in the electrophysiology laboratory to this day.
A secondary problem with the Chemical Theory of the Neuron has been the recent revelations, described below, that the simple movement of electron charges through organic molecular structures, particularly liquid crystalline structures is quite possible and readily occurs in commercially viable products (such as organic light emitting diodes).
Beginning at the start of the 20th Century, dramatic new knowledge appeared in the field of electro-chemistry that led to its maturation into separate fields related to;
This expanding knowledge base led to startingly different interpretations of the operation of neurons.
Nernst defined the first widely accepted law of chemical diffusion within a fluid environment. The fundamental premise of Nernst was that a semi-permable membrane, of unspecified structure, allowed chemcial ions to penetrate the membrane and reach the opposite side. The rate of particle penetration could be directionally sensitive, resulting in the descriptive term, semi-permeable. In the absence of external electrical polarization of the electrolytes on the two sides, a difference in charged particle concentration would develop spontaneously over time, thereby establishing a polarization across the semipermeable membrane.
With formalization of the diffusion laws, it became clear that a continuous concentration gradient must exist throughout the interior of the semi-permable membrane to support diffusion of each species involved in penetrating the membrane.
Initially, and throughout its restating, the Chmeical Theory of the Neuron has remained a poorly framed null hypothesis. Its major weaknesses include;
A major weakness in the hypothesis was its limited scope in failing to recognize the multiple-electrolytic-chamber character of the neuron.
The hypothesis failed to recognize the presence of the actual active device within the neuron
The hypothesis failed to recognize the lurking variable, the electrolytic potential difference between the dendroplasm and the podaplasm
The original null hypothesis known as the Chemical Theory of the Neuron can be refulted at multiple levels based on the empirical record.
A series of key experiments provide more than adequate falsification of the chemical theory of the neuron.
1. The chemical theory is founded on the application of the Nernst Equation, and subsequent tweaks to it by Donner and by Goodman to make it satisfy individual empirical data samples.
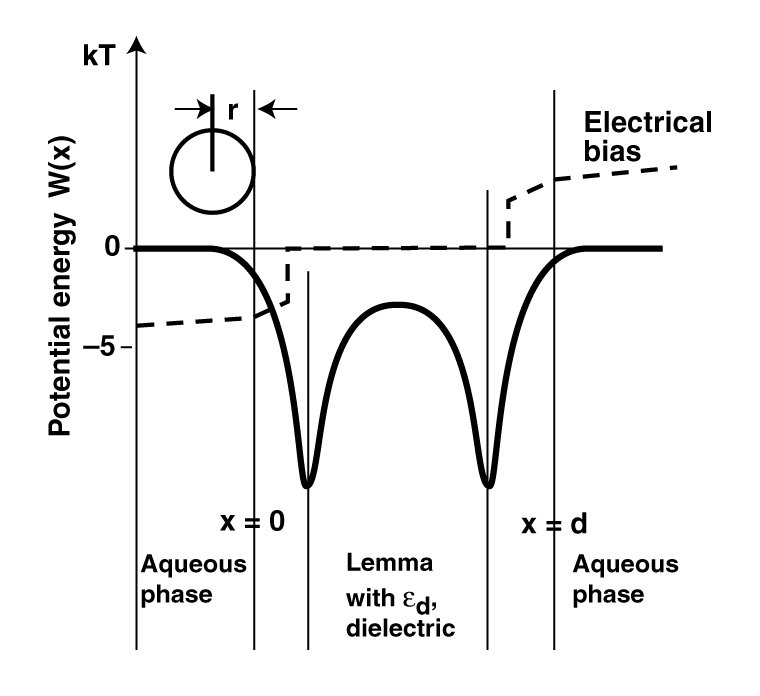
2. Application of the Nernst, Donner or Goodman equations
defines a "resting potential" determined primarily by the
relative concentrations of the sodium ion between the axoplasm
and the extra neural environment.
3. The chemical theory is based on the ability of sodium ions
to readily penetrate the axolemma of a neuron in timescales on
the order of microseconds.
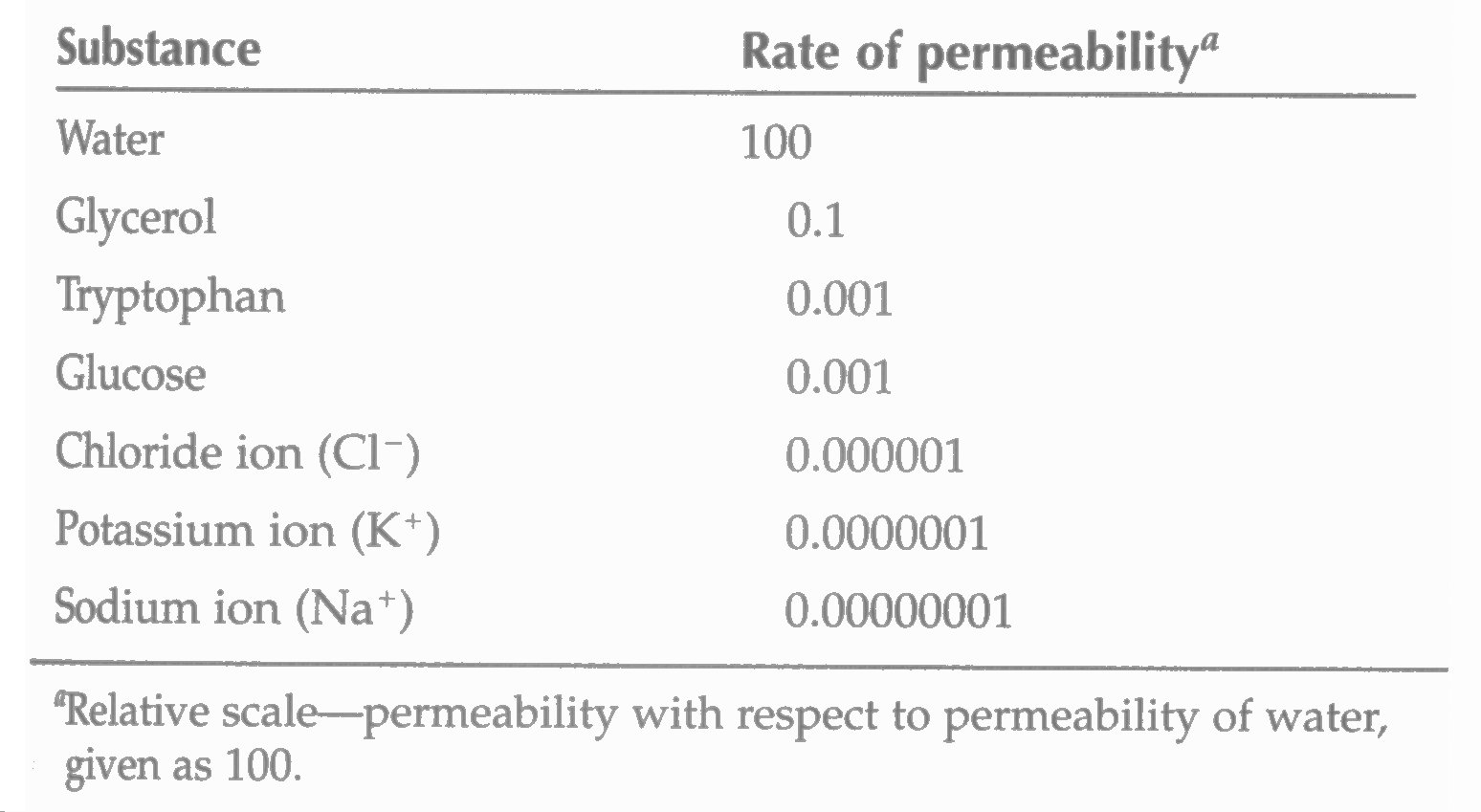
4. The chemical theory asserts the penetration of sodium ions
from the external matrix surrounding a neuron is critical to the
performance of the neuron.
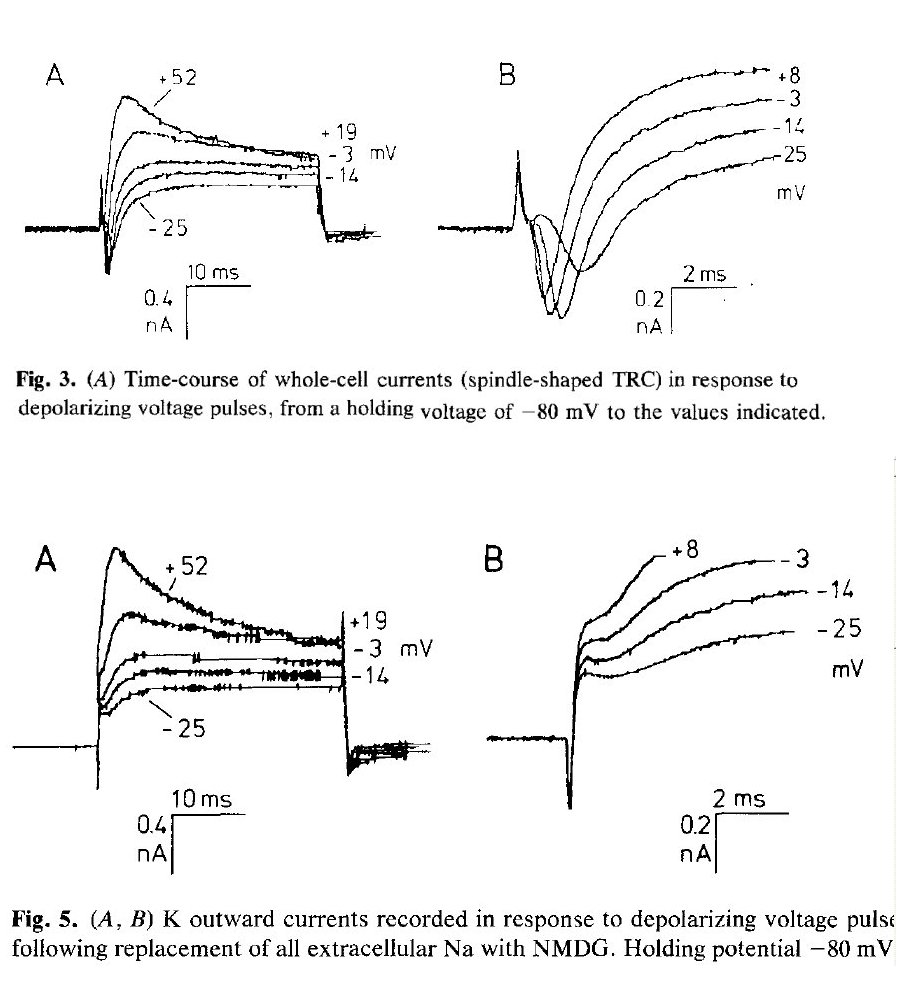
These experiments and data clearly refute the chemical theory of the neuron! It is a failed null hypothesis of the early 20th Century.
Fulton, J. (2010) Neuron and Neural System Processes. Chapters 4 in neuronresearch.net/neuron/document.htm
Return to the Neuron Research home page.