While the same form of sensory neuron is used in both the vestibular and cochlear parts of the labyrinth, they are used differently. In the vestibular portion, the free ends of the cilia of the neurons are tilted transversely by one of two motions. They may be tilted by the side to side motion of the fluids of the circular canals. Alternately, they may be tilted by the motion of calcified masses attacked to the ends of the cilia acting as inertial masses.
The cilia of the auditory sensory neurons operate differently. They are pushed axially by the motion of the surface acoustic wave traveling along the surface of the gel coating on the tectorial membrane. This action is resisted by the mounting structure supporting the sensory neurons. Note the bend in each sensory neuron immediately below the cuticular plate.
The axial force applied to the cilia of the audio sensory neurons distorts the cuticular plate of the neuron. This action will be illustrated below.
For the scope and top-level description of the SAW-based Electrolytic Theory of Hearing, see page 18 of Chapter 1 of the e-book "Processes of Biological Hearing"
The following material related to the sensory neurons is drawn from Chapters 4 & 5 of "Processes in Biological Hearing." A draft of this material can be accessed from the Home Page of this website.
The motion of the cilia is transferred to the piezoelectric proteins (actin) found within the cuticular plate. These proteins generate a quantum-mechanical electrical charge within the plate as they are distorted. The piezoelectric action is symmetrical. Motion in one direction causes a positive potential across the proteins. Motion in the opposite direction causes an opposite potential.
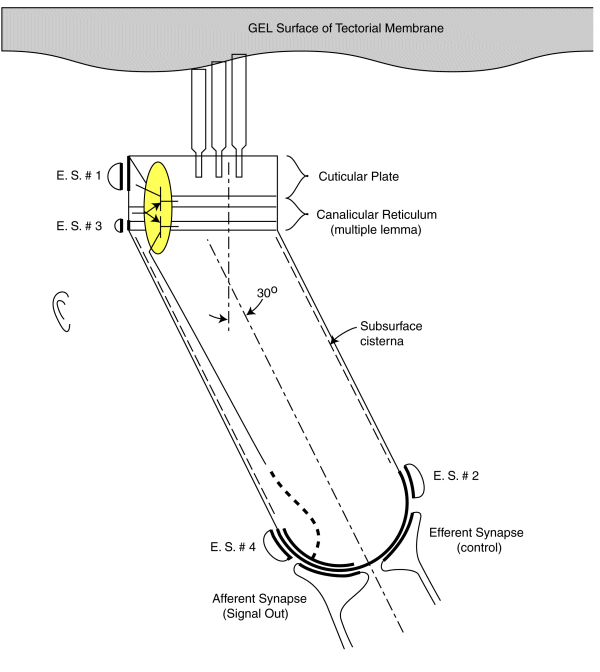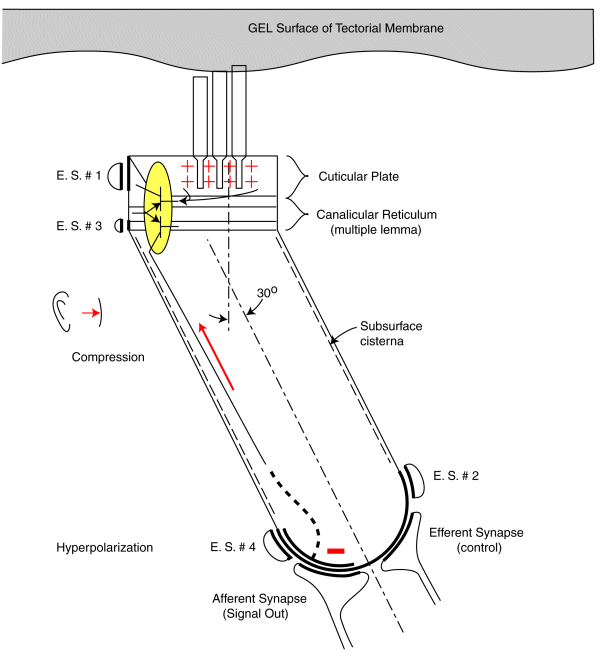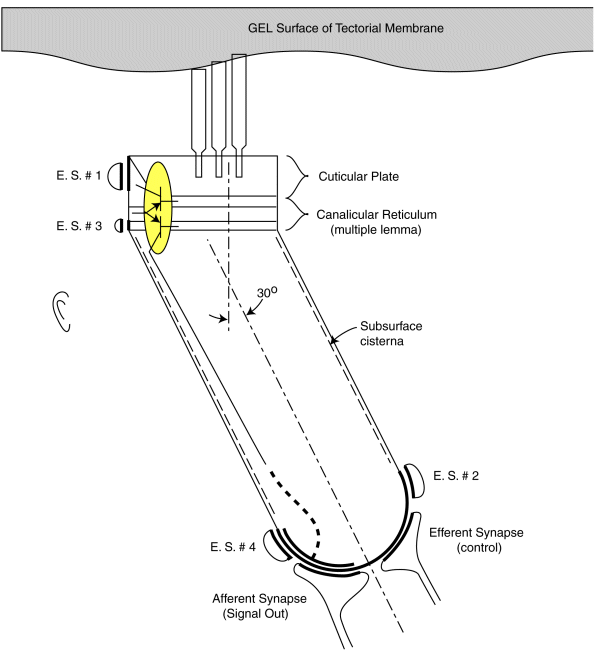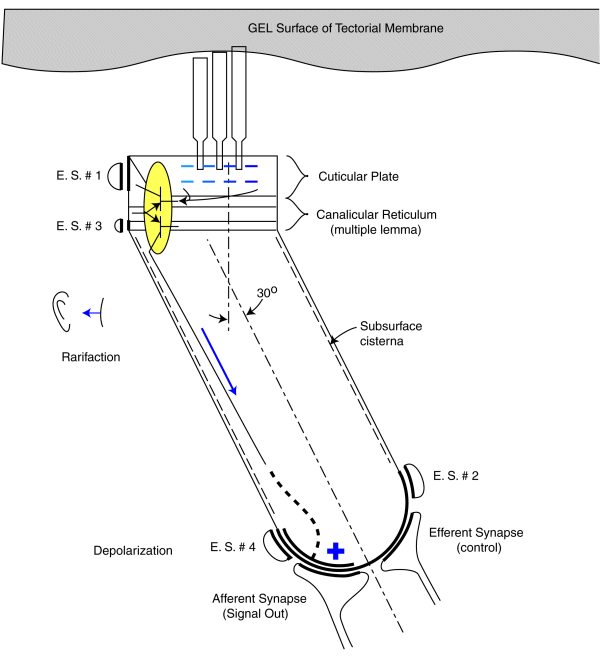
As noted earlier, each sensory neurons coontains a two stage electrolytic amplifier. The first stage is the adaptation amplifier that varies its amplification factor depending on the intensity of the signal exciting the neuron. The second stage is the distribution amplifier. It is used to lower the impedance at the axon of the neuron so that more synapses with other neuron can be supported. These amplifiers are contained within the structure known as the canalicular reticulum. The fact that this structure plays a role in the electrolytic operation of the neurons is widely recognized.
The electrolytic amplifiers of the sensory neuron are shown within the yellow oval in the following figure. The electrical power sources supporting these amplifiers are shown as electrostenolytic sources (E. S. # xxx).
The electrical charges generated by the distortion of the piezoelectric proteins in the cuticular plate are summed into a voltage across the capacitance of the cuticular plate. This voltage is applied to the input of the adaptation amplifier.
As a voltage is applied to the input of the adaptation amplifier, a current is caused to flow in the output of the distribution amplifier. This current flows down the axoplasm of the neuron and is collected by the neural arch (shown dashed). The current collected at the neural arch is transformed into a voltage (logarithmically) by the impedance associated with the electrostenolytic supply number 4. As a result, the neural arch exhibits a voltage that can be used to control the flow of current across the afferent synapses associated with the sensory neuron. This voltage is logarithmically related to the displacement of the cilia described above.
The amplification of the adaptation amplifier is controlled automatically and keeps the voltage at the pedicle of the axon to less than +/- 5 millivolts during normal operation. The cylindrical body of the sensory neurons are known to be electromotile. It expands when the voltage inside of them changes significantly. However, the neurons show very little electromotility for a potential change of less than +/- 5 millivolts. There are two other factors that limit the effect of any motility. First, the bandwidth of the sensory neurons is limited to frequencies below their integration frequency. This frequency is between 600 and 1000 Hz for humans. The neurons show negligible electromotility for frequencies significantly above this value. In addition, the angle between the direction of cilia motion and sensory neuron mounting further reduces the effect of this electromotility. The structure known as the subsurface cisterna appears designed to minimize the effect of electromotility on neuron operation.
The negligible mechanical feedback available at frequencies significantly above 600-1000 Hz makes the concept of a cochlear amplifier involving positive mechanical feedback based on electromotility unrealistic. This incompatibility is known as the RC paradox by the proponents of the mechanical cochlear amplifier.
The following figure illustrates the operation of the audio sensory neurons based on the above discussion. The animation applies to both the Outer Hair Cells and the Inner Hair Cells. The energy flowing along the gel surface of the tectorial membrane is shown as sinusoidal as would be expected at a Outer Hair Cell. The Inner Hair Cells would be expected to see a broader bandwidth signal as typified by a square wave near the basal end of the cochlea.
The energy is shown flowing past the cilia at about 6+ meters/sec. The peak-to-peak amplitude of the sine wave would be expected to be on the order of 10-20 nanometers. (The caricature does not represent any scale). The mechanical sine wave amplitude is not large enough to cause any significant neuron electromotility at physiological levels. Still, the amplitude of the sine wave is 30 to 40 dB greater than that measured at the basilar membrane (not shown and acting as an inertial mass). This factor suggests the auditory system is very efficient in transferring acoustic energy to the sensory neurons with minimal energy wasted in moving the basilar membrane or the bulk of the tectorial membrane.
A positive pressure (compression) at the tympanic membrane is known to cause a depolarization of the pedicle potential (equivalent to a current flowing into the pedicle (shown in red). Similarly, a negative pressure (rarifaction) at the tympanic membrane is known to cause a hyperpolarization of the pedicle potential (equivalent to a current flowing away from the pedicle (shown in blue).

The effect of efferent signals applied to the sensory neurons via the efferent synapse is not shown in this animation.
Go to the next animation in the operational chain. XXX NOT ACTIVE YET
Go to the roadmap of available animation of hearing. XXX NOT ACTIVE YET
Return to the hearing website home page.
Antoli-Candela, F & Kiang, N. (1978) Unit activity underlying the N1 potential In Naunton, R. & Fernandez, C. eds. (1978) Evoked Electrical Activity in the Auditory Nervous System. NY: Academic Press pg 165-191